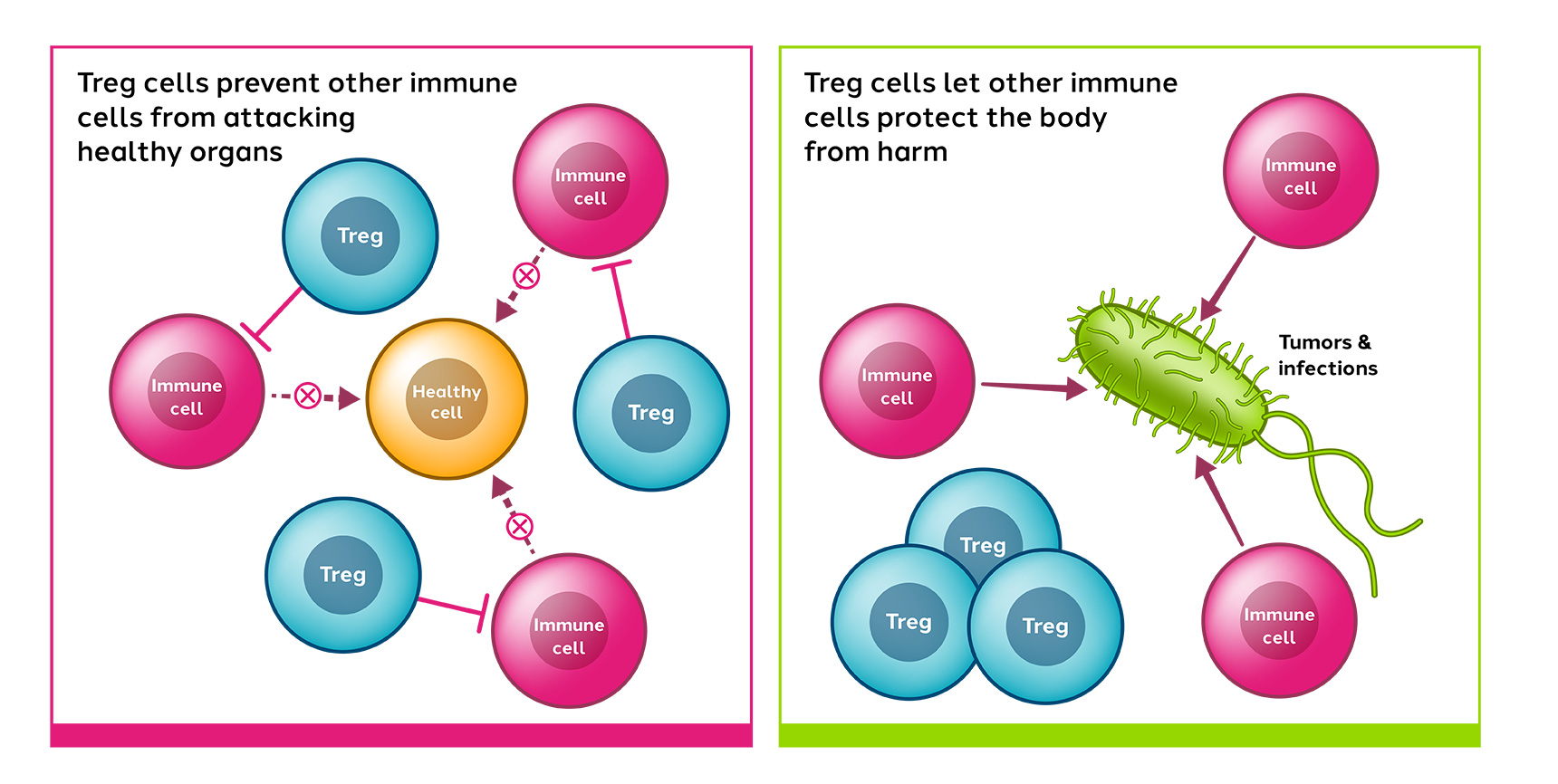
Tregs are a subset of immune cells with the ability to control and reduce the body’s immune reactions. They play a crucial role in maintaining immune homeostasis (keeping the immune system balanced), preventing undesirable immune reactions and can maintain immune tolerance. Treg cells have also been demonstrated to promote tissue repair and regeneration.
Dysregulation of Treg cell activity causes immune imbalance and can result in the loss of tolerance, autoinflammation and autoimmune diseases.

We expect that the resulting CAR-Treg cells will recognize and accumulate in specific tissues where this antigen is being expressed and an immune-mediated disorder is occurring. In doing so, we hope to leverage the Tregs’ multiple mechanisms of action to suppress inflammation in affected tissues.

TX200 is currently being evaluated in a clinical study in the area of renal transplantation, which we view as an opportunity to prove the biological approach and the value of the CAR-Treg platform. In March 2022, we were the first known company to dose a human patient with an engineered CAR-Treg therapy.
Learn more on our renal transplant program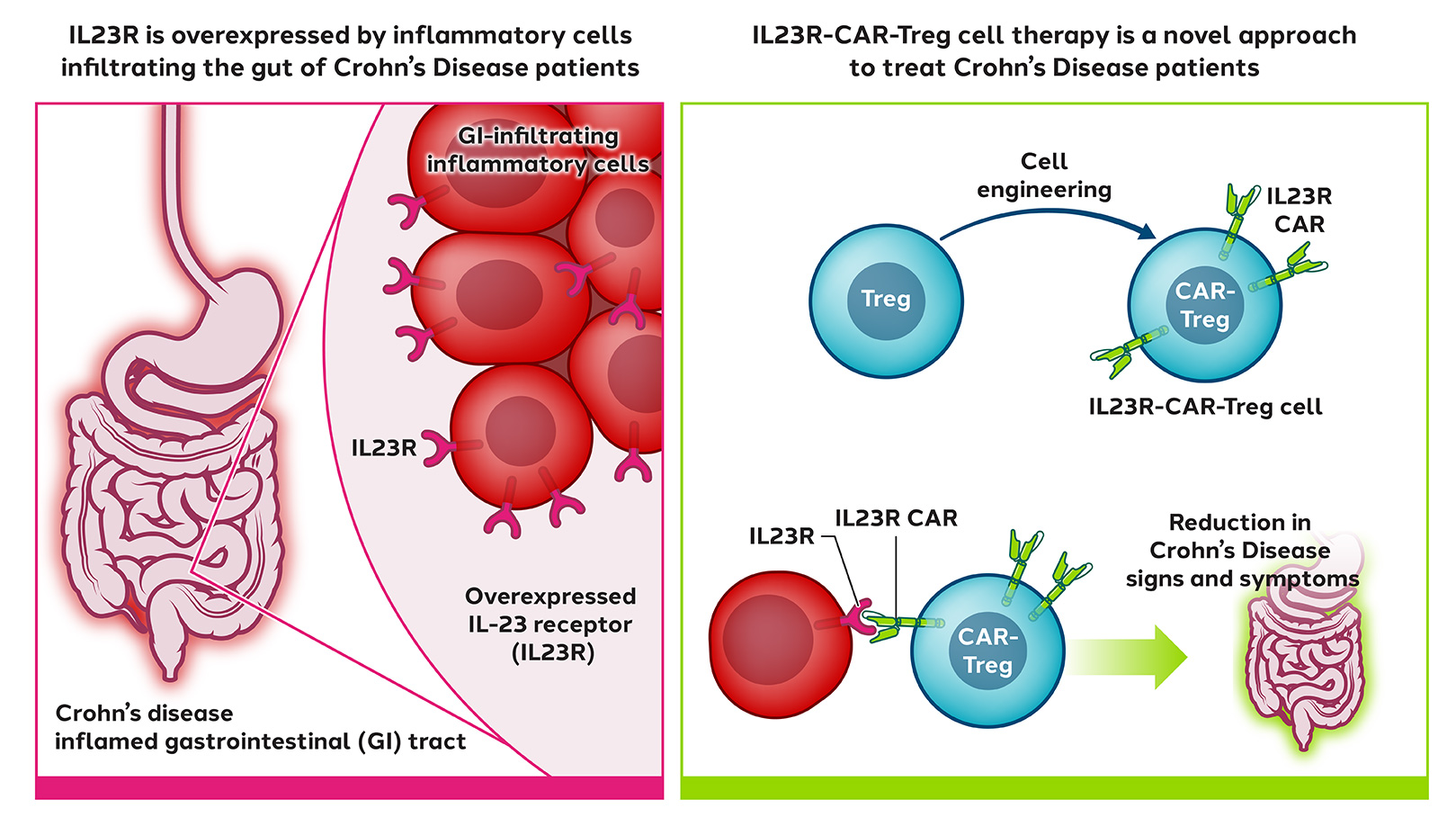
In inflammatory bowel diseases (IBD) such as Crohn’s disease, where improved treatments are needed, inflammation is localized in the gastrointestinal tract.
Sangamo has engineered Tregs with a CAR targeted to IL23R, a protein found in the gastrointestinal tract and overexpressed by inflammatory cells in Crohn’s disease patients.
When infused into the patient, CAR-Tregs are expected to migrate to the gastrointestinal tract, engage IL23R, and suppress local inflammation.
preclinical data from our IBD programWe are also progressing in our preclinical development of a wholly owned CAR-Treg program to treat multiple sclerosis (MS), an autoimmune disease of the central nervous system. Similar to our IBD program, our MS product candidate comprises autologous Treg cells engineered to express a CAR designed to recognize an antigen relevant to MS, allowing the resulting CAR-Tregs to localize and activate in the central nervous system.
preclinical data from our MS program
CAR-Tregs engineered by Sangamo using zinc finger technology are positioned to be the new frontier in cell therapy to potentially address inflammatory and immune-mediated diseases.
We believe that allogeneic therapies may be the future of cell therapy and could overcome the challenges of autologous approaches, such as scale and manufacturing.
Sangamo’s strategy is to first evaluate first-in-human proof-of-concept in an autologous study, and then potentially move to allogeneic approaches to address scalability. This may unlock tremendous potential for addressing various significant autoimmune indications, such as rheumatoid arthritis or diabetes.

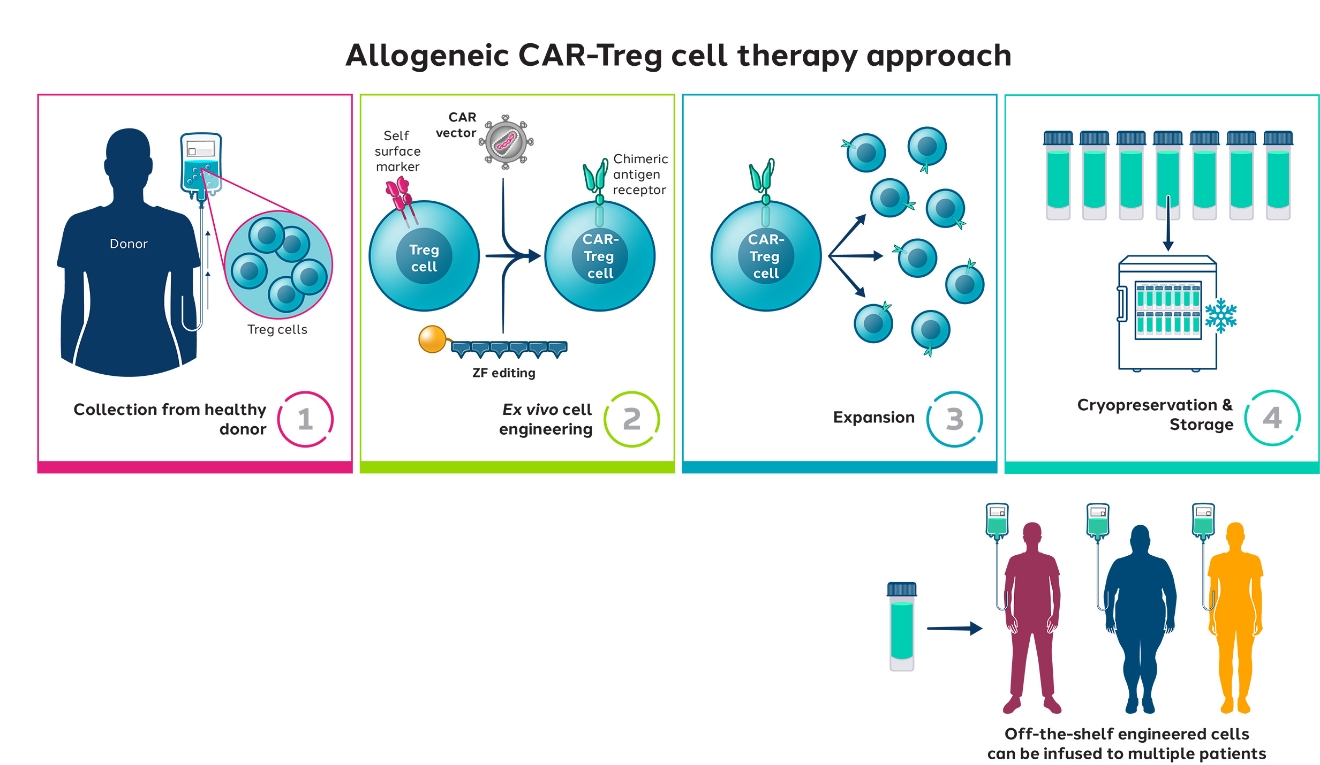
We are investigating various approaches to produce allogeneic CAR-Tregs, including the use of induced pluripotent stem cells (iPSCs) which can be differentiated into regulatory T cells in vitro under appropriate conditions.